CHAPTER 6: THE CHEMICAL COMPOSITION OF TEKTITES
RELIABILITY OF ANALYSES
Barnes (1939) compiled the tektite analyses available up to that time. Of these, the analyses prior to 1930 are not generally useful; many even lack TiO2. Of the analyses made in the 1930s the two bediasite analyses reported by Barnes (1939) are to be rejected; the MgO/CaO ratio in them is highly discordant with all other analyses. The analyses of F. Raoult, reported by Lacroix in various papers of this period, are comparable with good modern wet-chemical analyses.
In most wet-chemical analyses, there is a tendency to overstate the content of water and ferric iron. Friedman (1958) and Gilchrist et al. (1969) showed that water is present at the level of 0.01% (or less); it is thus below the limit of detection by wet-chemical methods. The ferric/ferrous ratio was found to be 0.05 for bediasites by Thorpe et al. (1963); values much over 0.15 are rare in modern analyses.
With respect to the trace elements, the work of Cohen (1959) on lithium and rubidium has been criticized by Schnetzler and Pinson (1963). The value of 7700 ppm for barium in moldavites is startling and very important if correct (Vorob'yev, 1960a); unfortunately accompanying values for barium in indochinites (Vorob'yev, 1959b) exceed those of other workers by a considerable factor.
CENTRAL COMPOSITION
Table
V gives the chemical composition of a representative tektite, in
particular an australite. For most elements, it is taken from S.R.
Taylor (1966), australite No. 28, 11947D. This australite is closely
similar to the normal australite of Chapman and Scheiber (1969), No.
45, AN 99. Where Taylor does not have data, the gaps have been filled
with other australite analyses as shown in the notes, or, in one or two
cases of non-critical elements, with other kinds of tektites.
TABLE V. Central tektite composition
|
Element |
Concentration (ppm, except as noted) |
Authority |
|
H |
6 |
Friedman (1958) |
|
He |
4.7 x 10-12 g/g |
Reynolds (1960) |
|
Li |
46 |
Taylor (1966) |
|
Be |
1.3 |
Vorob’yev (1964) |
|
B |
15 |
Chapman and Scheiber (1969) |
|
C |
97 |
Petersilye et al. (1968) |
|
N |
– |
– |
|
O |
482,000 |
Taylor (1966) |
|
F |
– |
– |
|
Ne |
1.5 x 10-13 g/g |
Reynolds (1960) |
|
Na |
11,000 |
Taylor (1966) |
|
Mg |
14,400 |
Taylor (1966) |
|
Al |
73,500 |
Taylor (1966) |
|
Si |
329,000 |
Taylor (1966) |
|
P |
170 |
Taylor (1966) |
|
S |
100 |
Cuttitta (1963) |
|
Cl |
43 |
Becker and Manuel (1971) |
|
Ar |
0.00011 |
Reynolds (1960) |
|
K |
20,400 |
Taylor (1966) |
|
Ca |
21,300 |
Taylor (1966) |
|
Sc |
14 |
Taylor (1966) |
|
Ti |
4700 |
Taylor (1966) |
|
V |
81 |
Taylor (1966) |
|
Cr |
76 |
Taylor (1966) |
|
Mn |
680 |
Taylor (1966) |
|
Fe |
39,900 |
Taylor (1966) |
|
Co |
16 |
Taylor (1966) |
|
Ni |
15 |
Taylor (1966) |
|
Cu |
5.6 |
Taylor (1966) |
|
Zn |
9.6 |
Greenland and Lovering (1963) |
|
Ga |
6.0 |
Taylor (1966) |
|
Ge |
– |
– |
|
As |
– |
– |
|
Se |
– |
– |
|
Br |
0.18 |
Becker and Manuel (1971) |
|
Kr |
– |
– |
|
Rb |
96 |
Taylor (1966) |
|
Sr |
150 |
Taylor (1966) |
|
Y |
25 |
Taylor (1966) |
|
Zr |
204 |
Taylor (1966) |
|
Nb |
13 |
Taylor (1966) |
|
Mo |
0.6 |
Taylor (1966) |
|
Ru |
– |
– |
|
Rh |
– |
– |
|
Pd |
– |
– |
|
Ag |
1.0? |
Cuttitta et al. (1962) |
|
Cd |
– |
– |
|
In |
< 0.1 |
Taylor (1966) |
|
Sn |
1.0 |
Taylor (1966) |
|
Sb |
0.29 |
Taylor (1966) |
|
Te |
– |
– |
|
I |
0.22 |
Becker and Manuel (1971) |
|
Xe |
– |
– |
|
Cs |
3.7 |
Taylor (1966) |
|
Ba |
590 |
Taylor (1966) |
|
La |
60 |
Taylor (1966) |
|
Ce |
84 |
Taylor (1966) |
|
Pr |
11.5 |
Taylor (1966) |
|
Nd |
33 |
Taylor (1966) |
|
Sm |
46 |
Taylor (1966) |
|
Eu |
1.3 |
Taylor (1966) |
|
Gd |
55 |
Taylor (1966) |
|
Tb |
1.0 |
Taylor (1966) |
|
Dy |
4.3 |
Taylor (1966) |
|
Ho |
12 |
Taylor (1966) |
|
Er |
1.18 |
Taylor (1966) |
|
Tm |
0.4 |
Taylor (1966) |
|
Yb |
1.9 |
Taylor (1966) |
|
Lu |
0.36 |
Haskin and Gehl (1963) |
|
Hf |
4.0 |
Taylor (1966) |
|
Ta |
0.73 |
Ehmann (1963) |
|
W |
0.65 |
Taylor (1966) |
|
Re |
0.000072 |
Lovering and Morgan (1964) |
|
Os |
0.00050 |
Lovering and Morgan (1964) |
|
Ir |
0.00001 |
Ehmann (1963) |
|
Au |
0.0057 |
Baedecker and Ehmann (1965) |
|
Hg |
0.016 |
Showalter (1970) |
|
Tl |
0.1 |
Taylor (1966) |
|
Pb |
1.4 |
Taylor (1966) |
|
Bi |
– |
– |
|
Th |
13.4 |
Taylor (1966) |
|
U |
1.9 |
Taylor (1966) |
In Figs. 28 and 29, the composition is compared respectively with
that of the U.S.G.S. standard granite, G-1, and the standard basalt,
W-1, using the 1972 recommended values (Flanagan, 1973). (These rocks
are not necessarily representative; they are chemical standards, which
are useful here because they have been fully and carefully analyzed,
and because they give an adequate idea of the two principal rock types. ) The arrangement is the long form of the periodic table. The ratio of the element concentration, in grams per gram, to the concentration of the standard, is represented logarithmically by bars going upward for excesses, and downward for deficiencies.
It is seen that, particularly on the left side of the periodic table, the tektite central composition falls between the granite and the basalt; where the bars are upward in the granite, they are downward in the basalt, and vice versa. Exceptions are the volatile elements: hydrogen, and a large group on the right side of a table enclosed by a line. For these elements, tektites are deficient as compared with both standard rocks.

|
Figure28.GIF Size : 0.028 Kb Type : GIF |
Click below for larger view.

|
Figure29.GIF Size : 0.023 Kb Type : GIF |
Click below for larger view.

|
Figure30.GIF Size : 0.028 Kb Type : GIF |
Click below for larger view.

|
Figure31.GIF Size : 0.026 Kb Type : GIF |
Click below for larger view.

|
Figure32.GIF Size : 0.028 Kb Type : GIF |
Click below for larger view.

|
Figure33.GIF Size : 0.027 Kb Type : GIF |
Click below for larger view.

|
Figure34.GIF Size : 0.024 Kb Type : GIF |
Click below for larger view.

|
Figure35.GIF Size : 0.018 Kb Type : GIF |
Click below for larger view.

|
Figure36.GIF Size : 0.025 Kb Type : GIF |
Click below for larger view.

|
Figure37.GIF Size : 0.025 Kb Type : GIF |
Click below for larger view.

|
Figure38.GIF Size : 0.029 Kb Type : GIF |
Click below for larger view.

|
Figure39.GIF Size : 0.025 Kb Type : GIF |
THE PRINCIPAL FAMILIES OF TEKTITES
When careful studies are made, including microtektites and rare types of macrotektites, it is seen that the range within one strewn field is often much greater than the range from one strewn field to another. In Table VI are given the major element compositions of tektites from various strewn fields. In the major fields, the attempt is made to choose examples which show the range in silica content, and the corresponding changes in the other major elements. Table VII lists the corresponding minor and trace elements, where these have been determined.
The variety of tektite types is such that some kind of overview is needed. In Figs. 34 and 35, the numbers of the points correspond to numbers of the columns in Tables VI and VII. Fig. 34 diagrams the more important types of tektites within the Australasian strewn field. To construct the figure, the weight percent MgO was taken from the analysis, added to CaO and 0.05 SiO2; call the sum s. Then MgO/s is plotted vertically upward from the base of the triangle, and similarly for the other two directions. The central composition of Table V is that of the normal australite, No. 1, near the middle of Fig. 34. Normal philippinites (10) and normal indochinites (2) are nearby.
It is therefore logical to compare with an intermediate rock; Fig. 30 shows the comparison with the standard andesite, AGV-1 (Flanagan, 1973); it is obvious that this is a better match than either the basalt or the granite, but the deficiencies on the right side of the table remain conspicuous.
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|
SiO2 |
70.4 |
73.3 |
87.7 |
83.2 |
68.1 |
67.76 |
51.6 |
59.2 |
63.4 |
71.6 |
68.6 |
|
Al2O3 |
14.3 |
13.1 |
7.42 |
8.22 |
16.5 |
13.04 |
14.7 |
22.1 |
16.8 |
13.0 |
11.0 |
|
Fe2O3 |
|
|
1.38* |
3.20* |
|
|
|
|
|
|
|
|
FeO |
4.97 |
4.49 |
-- |
-- |
5.75 |
8.63 |
4.44 |
3.5 |
5.67 |
4.55 |
4.55 |
|
MgO |
2.20 |
2.00 |
0.68 |
2.82 |
2.96 |
7.95 |
21.4 |
5.8 |
3.69 |
2.42 |
2.43 |
|
CaO |
2.94 |
2.17 |
0.02 |
0.16 |
1.82 |
2.85 |
4.16 |
4.5 |
4.49 |
3.31 |
9.77 |
|
Na2O |
1.56 |
1.27 |
0.01 |
0.19 |
1.08 |
0.72 |
0.06 |
0.7 |
3.84 |
1.50 |
0.93 |
|
K2O |
2.49 |
2.36 |
1.62 |
1.72 |
2.52 |
1.34 |
0.06 |
0.3 |
1.08 |
2.47 |
1.77 |
|
TiO2 |
0.82 |
0.89 |
0.52 |
0.52 |
0.92 |
0.71 |
0.73 |
n.d. |
0.58 |
0.78 |
0.62 |
|
P2O5 |
|
|
|
|
|
|
|
|
|
|
|
|
MnO |
|
|
|
|
|
|
|
0.1 |
|
|
|
|
Physical properties |
|||||||||||
|
S.G. |
2.450 |
2.428 |
|
|
2.481 |
2.591 |
2.786 |
|
2.535+ |
2.451 |
2.535 |
|
|
|
|
|
|
|
|
±0.015 |
|
|
|
|
|
R.I. |
1.513 |
1.508 |
|
|
1.519 |
1.544 |
|
1.509 |
1.532 |
1.513 |
1.534 |
*All Fe as Fe2O3.
1. Normal australite (Chapman and Scheiber, 1969, No. 45).
2. Normal indochinite (Chapman and Scheiber, 1969, No. 52).
3. Darwin-Macedon glass (Chapman et al., 1967a, No. DG-5).
4. Darwin-Macedon glass (Chapman et al., 1967a, No. Macedon 3531).
5. Low-calcium, high-aluminum tektite (Chapman and Scheiber, 1969, No. 42).
6. High-magnesium tektite (Chapman and Scheiber, 1969, No. 11).
7. Bottle-green Australasian microtektite (Glass, 1972b, No. 84-1).
8. Normal Australasian microtektite (Cassidy et al., 1969, No. 43).
9. High Na/K (Chapman and Scheiber, 1969, No. 24).
10. Normal philippinite (Chapman and Scheiber, 1969, No. 48).
11. High-calcium philippinite (Chapman and Scheiber, 1969, No. 6).
This result is surprising, in a way, because the SiO2 content is 70.4% for the central tektite, and 72.6% for G-1, while for AGV-1 it is 59.0%. We might thus have expected the tektite to be close to the granite than to the andesite, because it is well known that the chemical composition of igneous rocks is largely determined by the silica content. However, it was pointed out by Mueller (1915) that tektites could be distinguished from terrestrial igneous rocks because the ratio (FeO + MgO)/Na2O + K2O) was higher for a given silica content. This ratio decreases with increasing silica content, so that Mueller was finding the above-mentioned result, namely that tektite composition resembles (for most elements) that of terrestrial rocks of lower silica content. (We refer here to the left side of the periodic table.) Suess (1933) comments similarly; so do Loewinson-Lessing (1935). Barnes (1939) used this fact as the basis for his conclusion that tektites are for the most part formed from terrestrial sedimentary rocks. Urey (1958a, b) commented similarly. The same idea was expressed by Cherry and his collaborators (Cherry et al., 1960; Taylor, 1960, 1962c; Cherry and Taylor, 1961; Taylor and Sachs, 1961; Taylor et al., 1961).
Later, Taylor suggested that sedimentary processes could enhance the silica content in the way required. In particular, Taylor studied the Henbury sandstone (see Fig. 31); here it is clear that sedimentary processes are enhancing the silica content, because some of the sandstone has as much as 94% SiO2 (Taylor and Kolbe, 1965). By proper choice of sandstone, Taylor was able to come close to the australite composition, both in silica content and in other non-volatile elements (Taylor, 1966).
|
|
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
|
SiO2 |
84.48 |
79.10 |
75.5 |
83.6 |
76.2 |
71.89 |
64.4 |
58.20 |
67.6 |
51.4 |
84.65 |
98.20 |
|
Al2O3 |
7.79 |
9.75 |
11.12 |
9.50 |
13.4 |
17.56 |
16.7 |
5.35 |
16.8 |
16.0 |
6.0 |
0.70 |
|
Fe2O3 |
0.21 |
0.07 |
0.29 |
0.01 |
0.27 |
0.27 |
|
-- |
|
|
0.62 |
0.53 |
|
FeO |
0.98 |
1.52 |
1.50 |
1.82 |
3.74 |
5.26 |
6.79 |
8.57 |
6.14 |
10.5 |
1.96 |
0.24 |
|
MgO |
1.72 |
1.98 |
2.20 |
0.42 |
0.74 |
0.78 |
2.87 |
12.60 |
3.12 |
20.0 |
1.08 |
0.01 |
|
CaO |
1.90 |
2.89 |
3.40 |
0.40 |
0.74 |
0.45 |
2.49 |
11.40 |
1.49 |
2.75 |
0.30 |
0.30 |
|
Na2O |
0.20 |
0.69 |
0.48 |
1.19 |
1.63 |
1.28 |
1.48 |
0.50 |
2.04 |
0.40 |
0.24 |
0.33 |
|
K2O |
2.40 |
3.47 |
3.68 |
2.51 |
2.22 |
1.60 |
3.70 |
0.92 |
1.87 |
0.12 |
1.97 |
0.02 |
|
TiO2 |
0.22 |
0.57 |
0.31 |
0.42 |
0.74 |
1.05 |
0.90 |
|
0.52 |
0.50 |
0.65 |
0.23 |
|
P2O5 |
0.03 |
|
|
0.03 |
0.05 |
0.04 |
|
|
|
|
0.04 |
|
|
MnO |
0.05 |
|
|
|
|
|
0.09 |
0.34 |
|
|
0.0047 |
|
|
Physical properties |
|
|||||||||||
|
S.G. |
|
2.331 |
2.366 |
2.303 |
2.384 |
2.416 |
|
|
2.484 |
|
|
|
|
R.I. |
|
1.491 |
1.492 |
-- |
1.4962 |
1.5076 |
|
|
1.519 |
1.5956 |
|
|
12. High-silica moldavite (Schnetzler and Pinson, 1964a, No. T 4574).
13. Central moldavite (Bouška and Povondra, 1964, No. 3).
14. Low-silica moldavite (Philpotts and Pinson, 1966, No. T 5296f).
15. Georgia tektite (Cuttitta et al., 1967, No. GA. 2345).
16. Medium-silica bediasite (Cuttitta et al., 1967, No. B-6).
17. Low-silica bediasite (Cuttitta et al., 1967, No. B-90).
18. North American microtektite (Glass, 1973, No. 19).
19. North American crystalline spherule, like North American bottle-green (C. John and B.P. Glass, to be published in Geology).
20. Ivory Coast tektite (Chapman and Scheiber, 1969, No. 62).
21. Ivory Coast bottle-green microtektite (Glass, 1972b, No. 120).
22. Aouelloul crater glass (C.S. Annell, unpublished).
23. Libyan Desert glass (Spencer, 1939), average.
TABLE VII: Tektite families: trace elements (ppm) - Part A
(see Table VI for explanation of column numbers)
The discrepancy in hydrogen (i.e. water content) was commented on by Suess (1900, p. 247); in fact, the field test to distinguish a tektite from an obsidian is to heat it with a blowtorch or blowpipe; the tektite melts with at most a few bubbles, while the obsidian foams (La Paz, 1948). Terrestrial igneous rocks tend to have about 5000 ppm water, and sedimentary rocks several times as much. In an impact, however, the water may escape, at the price of turning the rock into a mass of bubbles (see e.g. Taylor and Kolbe, 1965).
On the right side of the periodic table, there are major discrepancies in many of the elements which are volatile at temperatures of the order of 1000° C. These discrepancies are difficult to measure by the usual methods of spectrochemical analysis. The elements are scarce; they volatilize at temperatures below those which are optimum for the metals on the left side of the diagram; and their spectra often have the important lines in relatively inaccessible parts of the ultraviolet. Hence these discrepancies tend to be overlooked. Nevertheless Preuss (1935) and Heide (1936b) noted that tektites are lower in copper, germanium, tin, and lead than their suggested terrestrial comparison material (Norwegian loam); similarly Taylor (1966) remarked on the deficiencies in copper, lead, tin, thallium, iridium, and bismuth compared with the Henbury impact glasses.
Similar discrepancies are conspicuous when lunar rocks are compared with terrestrial rocks of similar type. In Fig. 32 a basaltic clast, 14321.223 from Apollo 14 (Wänke et al., 1972) is compared with W-1. In Fig. 33 the central tektite composition is compared with lunar sample 12013, the only lunar sample of acidic composition for which substantial trace element data exist. The comparison is unsatisfactory in several respects; but it suggests that the systematic discrepancy in the volatile elements is removed.
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ag |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
B |
15 |
12 |
|
|
27 |
11 |
|
|
11 |
39 |
22 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ba |
410 |
365 |
300 |
400 |
390 |
410 |
|
|
355 |
400 |
380 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Be |
|
|
2.6 |
2.6 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Co |
13 |
11 |
4 |
34 |
13 |
56 |
|
|
46 |
13 |
18 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cr |
78 |
80 |
46 |
160 |
125 |
440 |
1500 |
|
235 |
105 |
105 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cs |
|
|
3.1 |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cu |
5 |
4 |
1.4 |
|
n.d. |
5 |
|
|
16 |
8 |
n.d. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Dy |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Eu |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ga |
|
|
<5 |
8.7 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ge |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
La |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Li |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mn |
820 |
730 |
50 |
245 |
910 |
1700 |
800 |
|
770 |
670 |
1700 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Nb |
|
|
18 |
19 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ni |
28 |
16 |
71 |
500 |
30 |
230 |
|
|
675 |
35 |
43 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pb |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Rb |
|
|
105 |
90 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Sc |
|
|
7.8 |
8.5 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Sr |
|
|
17 |
16 |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Th |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
U |
|
|
12-14* |
|
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
|
Ag |
|
|
<1 |
<1 |
2 |
|
600 |
|
|
|
|
|
B |
9 |
(Preuss, 1935) |
<10 |
<10 |
30 |
|
|
20 |
|
2 |
|
|
Ba |
|
|
715 |
440 |
420 |
|
|
650 |
|
420 |
|
|
Be |
2 |
(Preuss, 1935) |
1.9 |
2 |
5 |
|
|
|
|
-- |
|
|
Co |
-- |
|
6.7 |
11 |
12 |
|
|
19 |
|
20 |
|
|
Cr |
40 |
(Preuss, 1935) |
18 |
46 |
62 |
|
|
210 |
|
70 |
|
|
Cs |
|
|
1.4 |
1.7 |
2.3 |
|
|
|
|
1.4 |
|
|
Cu |
5.4 |
(Greenland and Lovering, 1962) |
3.2 |
10 |
14 |
|
|
12 |
|
9.5 |
|
|
Dy |
2.8 |
(Chase et al., 1963) |
|
|
|
|
|
|
|
-- |
|
|
Eu |
0.83 |
(Chase et al., 1963) |
|
|
|
|
|
|
|
-- |
|
|
Ga |
-- |
-- |
9.0 |
11 |
16
|
|
|
|
|
8 |
|
|
Ge |
-- |
-- |
|
|
|
|
|
|
|
-- |
|
|
La |
29 |
(Chase et al., 1963) |
<50 |
50 |
40 |
|
|
|
|
-- |
|
|
Li |
14 |
(Goldschmidt et al., 1933) |
14 |
22 |
24 |
|
|
|
|
12 |
|
|
Mn |
|
|
218 |
200 |
170 |
|
|
555 |
1100 |
340 |
|
|
Nb |
|
|
<10 |
18 |
22 |
|
|
|
|
10 |
|
|
Ni |
15 |
(Preuss, 1935) |
9.5 |
16 |
14 |
|
|
86 |
|
360 |
200 |
|
Pb |
6 |
(Tilton, 1958) |
|
|
|
|
|
|
|
|
|
|
Rb |
140 |
(Schnetzler and Pinson, 1963) |
80 |
72 |
54 |
|
|
|
|
66 |
|
|
Sc |
3 |
(Goldschmidt and Peters, 1932) |
7.8 |
10 |
16 |
|
|
|
|
12 |
|
|
Sr |
136 |
(Pinson et al., 1958) |
153 |
85 |
60 |
|
|
|
|
44 |
|
|
Th |
15.4 |
(Dubey, 1933) |
|
|
|
|
|
|
|
|
|
|
U |
2.2 |
(Starik et al., 1961; Adams et al., 1959; Dubey, 1933) |
|
|
|
|
|
|
|
|
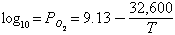 where P02 is in atmospheres, and T is the Kelvin temperature. The corresponding number in N/m2 is greater by a factor of 105. The relation yields 10-9.6 N/m2 (or 10-14.6 atmosphere) at 1100° C, about six orders of magnitude below terrestrial rocks at this temperature (Walter and Doan's value of 10-17.6 atmosphere for 800° C is a slip; it should be about 10-21.3 according to their unpublished charts). More measurements are needed. Vorob'yev (1959a) found magnetite spherules, often hollow, in surface cavities of Philippine tektites. Kleinman (1967) found magnetite inclusions some of which had nickel-free iron cores, as if the tektites had formed in equilibrium with metallic iron, which later oxidized (verbal suggestion by L.S. Walter). This would again suggest a low oxygen fugacity. Brett (1967) has noted a relation between the nickel content of metallic spherules in tektites and impact glasses, and the presence or absence of a halo of iron oxide in the surrounding glass. It appears that for impact glasses, a portion of the iron oxidizes and dissolves in the glass, leaving the spherule nickel-rich. For tektites, on the other hand, the nickel enrichment and the iron oxide halo are both missing. This result seems to imply that the oxygen fugacity in tektites is much lower than in impact glasses, so that they do not tend to oxidize the iron.
Make a Free Website with Yola.
|